Bromine (Br). Diagram of the nuclear composition and electron configuration of an atom of bromine-79 (atomic number: 35), the most common isotope of t
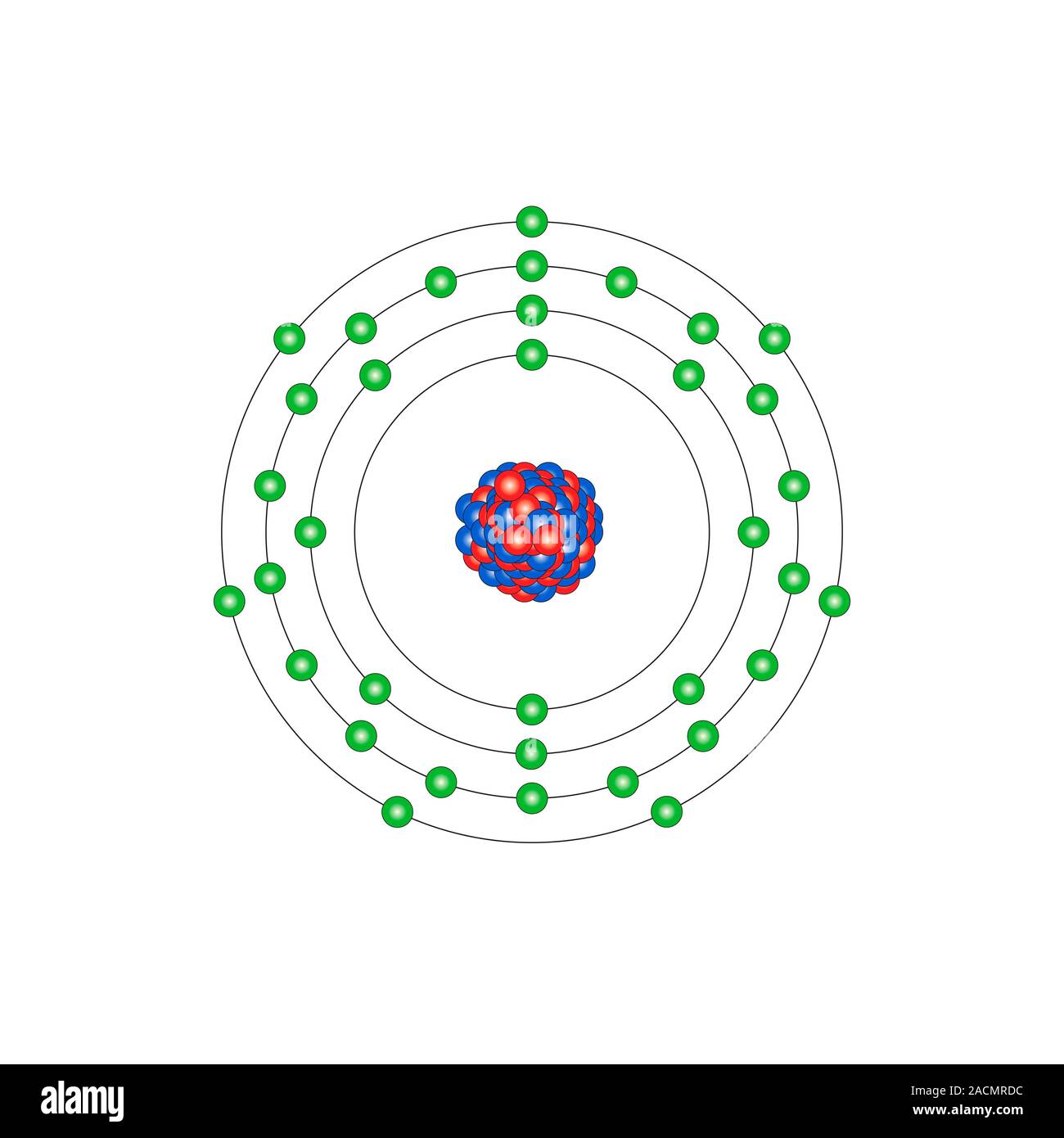
Image details
Contributor:
Science Photo Library / Alamy Stock PhotoImage ID:
2ACMRDCFile size:
60 MB (420.6 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
4579 x 4579 px | 38.8 x 38.8 cm | 15.3 x 15.3 inches | 300dpiDate taken:
2 May 2012Photographer:
Science Photo LibraryMore information:
Bromine (Br). Diagram of the nuclear composition and electron configuration of an atom of bromine-79 (atomic number: 35), the most common isotope of this element. The nucleus consists of 35 protons (red) and 44 neutrons (blue). 35 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The stability of an element's outer electrons determines its chemical and physical properties. Bromine is a halogen in group 17, period 4, and the p-block of the periodic table. In elemental form it is a red-brown, corrosive and toxic liquid (Br2) that boils at 59 degrees Celsius. Its main use is in fire retardant compounds.